
Example 4.1 Calculate the formula mass (FM) of NaOH using a table of atomic masses (AM). Therefore chemists often use the term formula mass to represent the total composition of such substances. For example, the molecular mass of ethanol, C2H5OH, is: (2×C)+(6×H)+(1×O) (2×12.0)+(6×1.0)+(1×16.0) = 46 When ionic compounds such as NaCl, Zn(NO3)2, andNH4Cl, are in the crystalline state or in solution form, they do not contain physically distinct uncharged molecular entities. The term applies only to compounds that exist as molecules, such as H2O, SO2, and glucose, C6H12O6. The molecular mass of a compound is the sum of the atomic masses of all the atoms in one molecule of the compound. of O) = (3×1.00)+(1×30.97)+(4×16.00) = 97.97 Once the actual formula of a chemical substance is known, the molecular mass can be determined in a manner similar to calculating the formula mass. For example, the formula mass of phosphoric acid, H3PO4, is 97.98 atomic mass units (amu), which is obtained by adding the atomic masses (taken from the periodic table) of the elements in one formula unit (i.e., 3 H + 1 P + 4 O). What is the relative molecular mass of ethanol To do calculations in Chemistry, we need to be able to count the number. Formula masses are relative since they are derived from relative atomic masses. The formula mass is based on the ratio of different elements in a formula, as opposed to the molecular mass, which depends on the actual number of each kind of atom (compare section 6.2, “Empirical Formula”). The reason that Ethanol had the lowest ethanol change at -478.74 is due to the fact of. Hexanol required the most amount of energy to break the bonds due to its high Molar Mass and long carbon chains, this is the reason that it had the highest Enthalpy change of -2400.
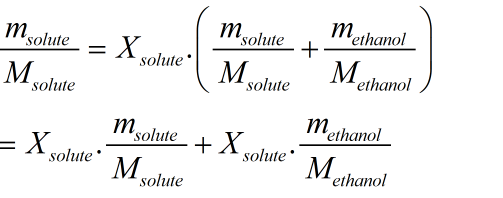

The formula mass of a compound is the sum of the atomic masses of all the atoms in a formula unit of the compound, whether it is ionic or molecular (covalent). The lowest Enthalpy was Ethanol with at -478.74 with a molar mass of 32.04. But there is a slight difference between the two terms, as explained below.

For more information, please read the general European excise regulations and check with your local excise authorities.Many chemists use the terms formula mass and molecular mass interchangeably when dealing with chemical compounds of known formula. It depends on the local national legislation whether a special permit is required and under which conditions a recipient may receive ethyl alcohol. We would like to point out once again that the individual EU member states each have their own regulations regarding the acceptance of ethanol. Properties of Solutions (Chapter Thirteen) The density of ethanol is 0.789 g/mL and the density of water is 1.0 g/mL. sec-butyl amine including 2 optical isomers. How many moles are in 32. We will look at compounds containing polyatomic ions, and also hydrate compounds. the molecular mass increases with 42 units. Molar mass is a quantity that is very similar to molecular mass, molecular weight, formula mass, and formula weight. Denatured ethanol can be delivered under excise exemption, provided that the national authorities accept the added product as denaturant. How many secondary amines have the molecular formula C4H11N. In this case, you as the consignee must present a permit from your national authorities to receive ethyl alcohol under excise suspension. Our ethanol can be delivered undenatured, without any additional products. We place a high priority on complying with the regulations of the EU member states in which we operate. A solution of ethanol, C2H6O, is prepared by dissolving 14.0 g C 2H6O in 100.0 g water. In one to two sentences, make and justify a claim about the number of moles of ethanol the chemist will use.

A chemist will use a sample of 30 g of ethanol (CH3CH2OH) in an experiment. Concentration: femtomolar, picomolar, nanomolar, micromolar, millimolar, molar. Within the EU and UK, excise tax rates vary between member states and can be a multiple of the retail price. Use the table of molar masses to complete the activity. Ethanol is subject to excise duty, national authorities strictly control all shipments.


 0 kommentar(er)
0 kommentar(er)
